ETAS®Study Results
Brain Function Improvement
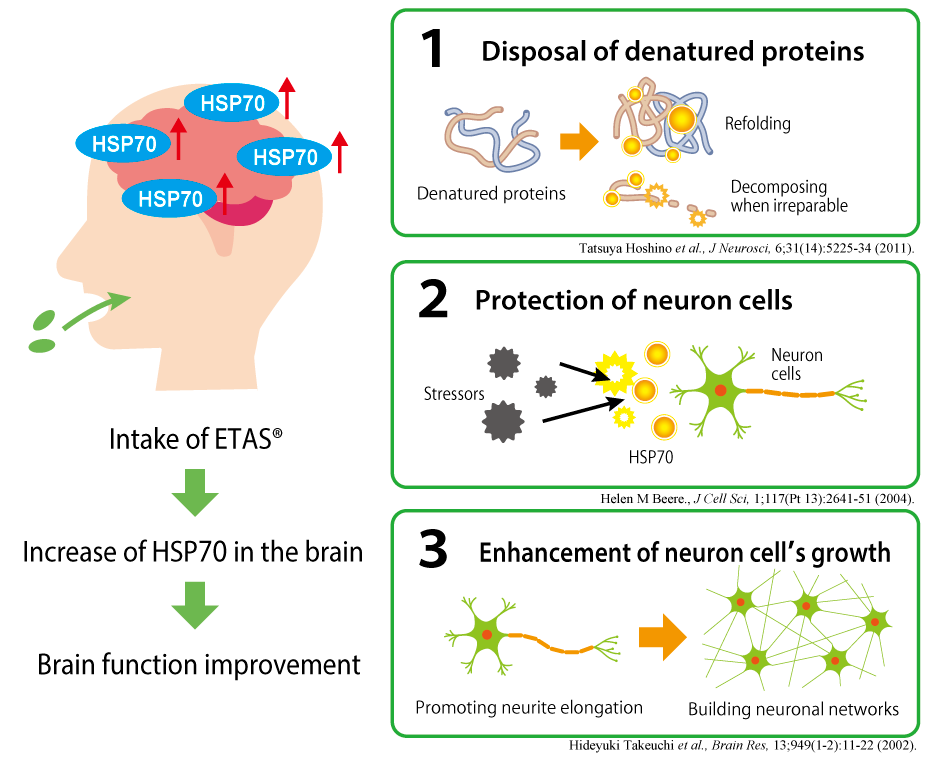
Three actions on brain function expected
from HSP70 induction effect of ETAS
RESEARCHImprovement in brain tissue parameters related to the circadian rhythm
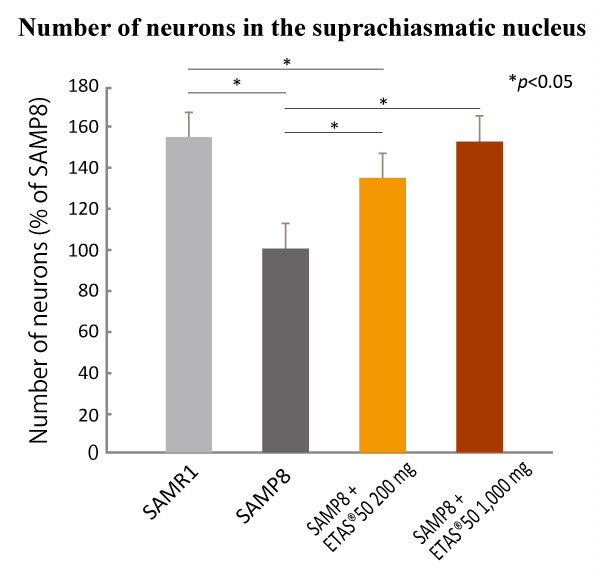
ETAS® maintained the number of neurons in the suprachiasmatic nucleus (SCN).
ETAS® intake kept the number of neurons which gradually decreases in the brain of Senescence-Accelerated Mice
(SAM). In addition, it is proven that melatonin receptors that have functional roles in sleep-onset and the
circadian rhythm*1 were regulated properly.
These results allow us to expect ETAS® to help adjusting the
disrupted circadian rhythm.
Yin-Ching Chan et al., Nutrients, 11(7): 1631 (2019)
Non-clinical study (in vivo)
Animal: 3-month-old male SAMR1 and SAMP8 mice
Groups: SAMR1 (n=8), SAMP8 (n=8), low-dose ETAS®50/SAMP8 (n=8), and high-dose ETAS®50/SAMP8 (n=8)
Doses and period: ETAS®50 200 and 1,000 mg/kg/day for 12 weeks
Evaluation: Neuron number in the suprachiasmatic nucleus (SCN) and expression levels of hypothalamus
melatonin receptors 1 and 2 (MT1 and MT2)
Results
ETAS®50 dose-dependently reduced the neuron loss in the SCN and properly regulated the expression of hypothalamus melatonin receptors adequately.
*1 Circadian rhythm: an internal rhythm that is regulated to be a twenty-four-hour cycle by environmental insults such as light-dark change, temperature, and meals. The circadian rhythm is controlled by the body’s clock located in the hypothalamus.
RESEARCHActive avoidance learning improved by better brain function
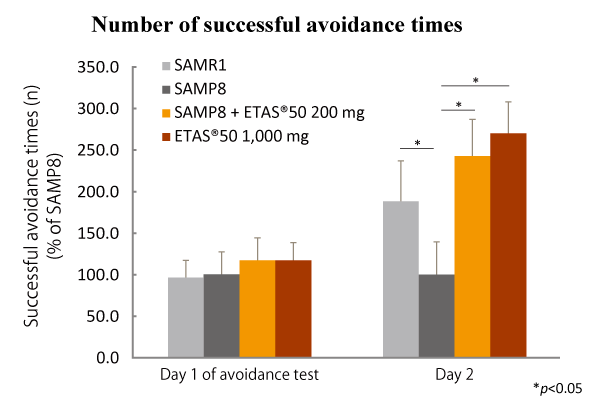
ETAS® improved the brain function to sense and avoid a danger.
Senescence-Accelerated Mice showed improved abilities to see and avoid a danger after taking ETAS® for 12 weeks
dose-dependently. It is also showed that the accumulation of amyloid β*2 in the brain was suppressed
by ETAS® intake.
These results suggest that ETAS® could improve the impaired brain function.
Yin-Ching Chan et al., Nutrients, 11(7): 1631 (2019)
Non-clinical study (in vivo)
Animal: 3-month-old male SAMR1 and SAMP8 mice
Groups: SAMR1 (n=8), SAMP8 (n=8), low-dose ETAS®50/SAMP8 (n=8), and high-dose ETAS®50/SAMP8 (n=8)
Doses and period: ETAS®50 200 and 1,000 mg/kg/day for 12 weeks
Evaluation: Cognitive performance using active avoidance test*3 and accumulation of amyloid β
(Aβ) in the whole brain tissue and hippocampus
Results
ETAS®50 dose-dependently contributed to accomplishing good outcomes in the active avoidance test. Furthermore, ETAS®50 significantly lowered the expression levels of Aβ2 precursor protein and BACE-1 involved in Aβ generation, resulting in significant suppression of Aβ accumulation in the whole brain tissue and hippocampus.
*2:amyloid β: Its abnormal production and accumulation in the brain are ascribed as the cause of
developing Alzheimer’s disease.
*3:Active avoidance test: a test in which the tested animal learns to avoid an aversive stimulus followed by
precautions (light or sound) by changing locations beforehand.
RESEARCHCognitive performance improvement offered by increase of HSP expression in the hippocampus
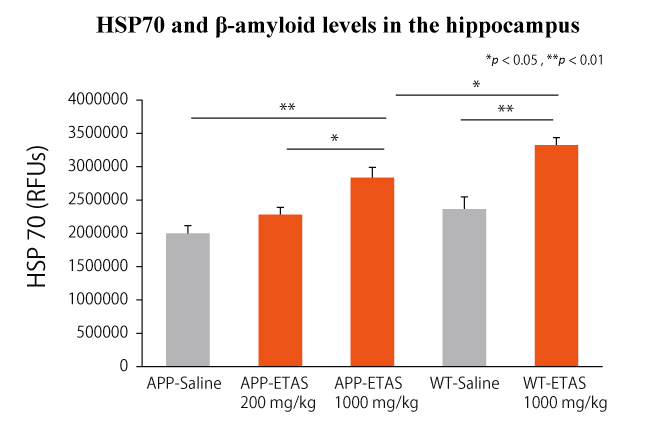
ETAS® improved cognitive function by increasing HSP70 in the hippocampus of Alzheimer’s disease animal models.
Alzheimer’s disease model mice showed improved performance on the Morris Water Maze (MWM)*4 task after taking ETAS® for a month. In the hippocampus, the expression of HSP70 has increased in a dose-dependent manner of ETAS®, and the accumulation of amyloid-β and tau proteins and production of caspase-3 were also shown to be suppressed. These findings suggest that ETAS® has the potential to help maintain healthy cognitive abilities.
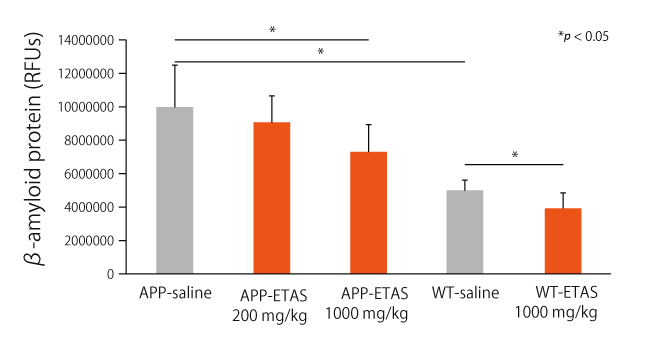
Zhanglong Peng et al., Journal of Immunology Research, 10;2021:8121407 (2021)
Non-clinical study (in vivo)
Animal: Male and female Alzheimer’s disease model mice overexpressing APP*5 and presenilin at 6 to 8 weeks old
Groups: Wild type (n = 10), high-dose ETAS®50/Wild type (n = 10), Model mice (n = 10), low-dose ETAS®50/Model mice (n = 10) and high-dose ETAS®50/Model mice (n = 10)
Doses and period: ETAS®50 200 and 1,000 mg/kg/day for a month
Evaluation: Assessment of spatial learning and memory using the MWM, amount and expression of HSPs, accumulation of soluble and insoluble β-amyloid and tau protein, and amount of caspase-3 in the hippocampus
Results
ETAS®50 significantly upregulated the expression of HSP70 in the hippocampus. Furthermore, escape latency and residence time increased significantly, while accumulation of amyloid-β and tau protein as well as caspase-3 levels decreased significantly in a dose dependent manner by ETAS®50 intake.
*4 Morris Water Maze (MWM): One of the most widely used tasks in behavioral neuroscience to evaluate spatial learning and and memory abilities.
*5 APP : Amyloid Precursor Protein