AHCC®Standardized extract of cultured mushroom mycelia
- HOME
- AHCC®

Functional Ingredient with Scientific Evidence of its Efficacy in Immune Modulation
Since its development in 1986, the efficacy in immune modulation and the safety of AHCC®, a standardized extract of cultured Lentinula edodes mycelia, have been researched by numerous researchers and health professionals at about 100 medical institutes and universities in the world.
Study results
Scientific Publications
More than 100 universities and research facilities worldwide have conducted studies on AHCC® and published more than 100 academic papers.
.What is AHCC®?
Raw Material
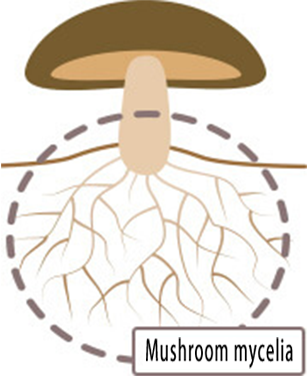
Active Components
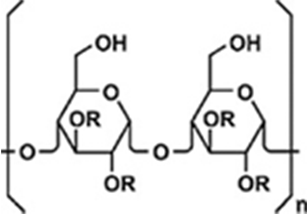
.Features of AHCC®
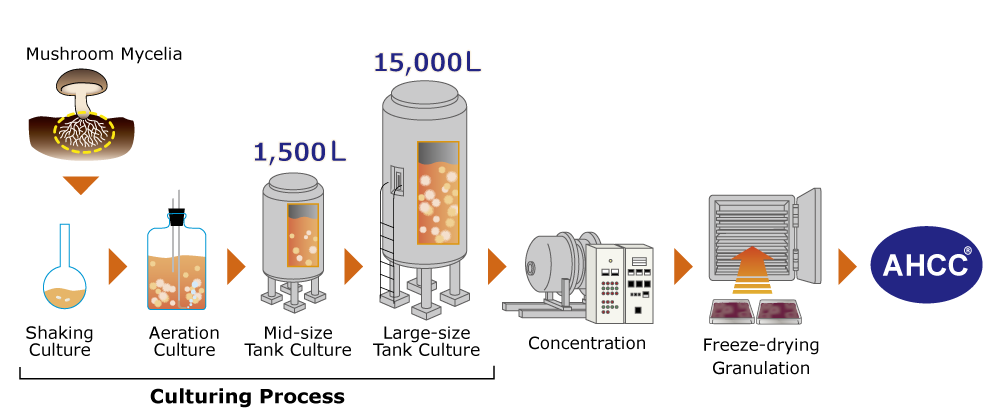
The proprietary long-term liquid culturing process enables us to produce consistent and standardized quality of AHCC® by culturing the specialty strain of mycelia, which we found by screening a vast range of basidiomycetes in 1987. This unique process produces special components of AHCC® that are different from other mushroom products in the market.
.Manufacturing process of AHCC®
.Safety of AHCC®
Toxicity Assay
・Acute oral toxicity test:LD50 > 12,500 mg/kg
・Subchronic oral toxicity study:NOAEL = 3,000 mg/kg/day
・Reverse mutation test (Ames test): Negative
・Micronucleus test: Negative
Safety Study
Human safety test (Phase I):9,000 mg/day
No serious adverse effect has been reported since its launch in 1987.
.Certification
Certified as Halal
AHCC® has been certified as Halal by their authorized certification body.
