AHCC®Study results
Cancer
RESEARCHRecurrence prevention After Curative Hepatectomy of Hepatocellular Carcinoma
AHCC® improved recurrence-free survival rate and hastened nutritional recovery in post-operative patients
Curative hepatectomy is the standard treatment for advanced hepatocellular carcinoma. It is very important for post-operative patients to prevent recurrence and recover their nutritional conditions. The results of this study showed that AHCC® intake helped lower the recurrence rate by 10 % compared to the historical data. Based on the result, AHCC® could be beneficial for patients with hepatocellular carcinoma as a safe adjuvant therapy after surgery.
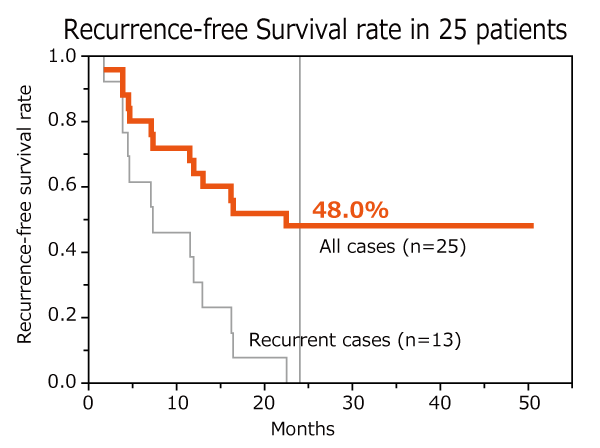
Toshiya Kamiyama Integrative Cancer Therapies, 21: 15347354211073066 (2022)
Clinical study
Design: non-randomized open label study
Subject: 29 patients after curative hepatectomy of hepatocellular carcinoma
Dose: AHCC® 3 g/day
Endpoints: recurrence-free survival rate and nutritional recovery
Results
AHCC® intake for two years after curative hepatectomy lowered the recurrence rate by 10 % compared to the historical data and hastened recovery without any adverse event.
*The specified clinical trial in Japan (approval no. jRCTs011180006)
RESEARCHPrognosis improvement in postoperative hepatocellular carcinoma patients
AHCC® extended the no-recurrence period and increased the overall survival rate.
After removing cancer by surgery, it is very important to have a good prognosis*1 and prevent recurrence.
Postoperative Hepatocellular Carcinoma patients who kept taking AHCC® showed longer overall survival duration and lower recurrence rate than those who went without AHCC®. These results suggest that AHCC® improves the prognosis after surgery.
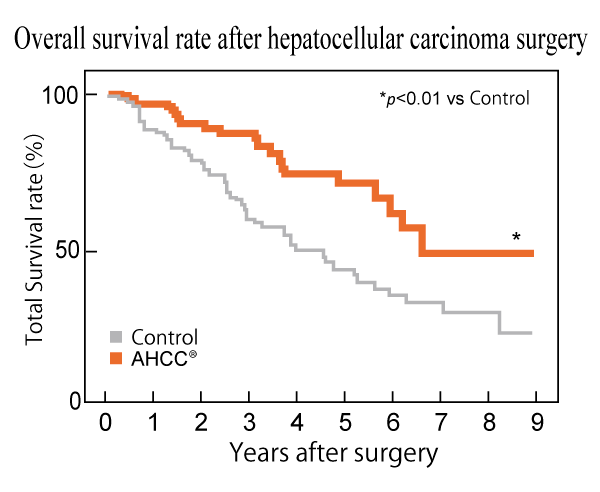
Yoichi Matsui et al., Journal of Hepatology, 37(1): 78-86 (2002)
Clinical study
Design: A prospective cohort study
Subject: 269 patients with postoperative hepatocellular carcinoma (HCC)
Groups: AHCC® (n=113) and Control (non-AHCC®) (n=156)
Dose: AHCC® 3 g/day
Endpoints: Recurrence rate, overall survival duration after surgery, and liver function-related parameters for 9 years
Results
AHCC® intake increased overall survival rate and extended no recurrence period, suggesting that AHCC® might ameliorate postoperative prognosis.
*1: Prognosis: medical prospect of survival and recovery from a disease anticipated from the current status of progression and effectiveness of the treatments.
RESEARCHReduction of side effects of adjuvant chemotherapy for breast cancer
AHCC® alleviated neutropenia, depletion of white blood cells, common side effects of adjuvant chemotherapy
Chemotherapy is a common treatment for cancer therapy; however, its side effects are associated with reduction of anticancer drugs and discontinuation of the treatment, frequently resulting in unsatisfactory outcome from chemotherapy.
In the study that examined whether AHCC® alleviates side effects of adjunctive chemotherapy for breast cancer, AHCC® improved neutropenia and decreased the use of antineutropenia agents.
It is expected that AHCC® is available to adequately exert the efficacy of chemotherapy.
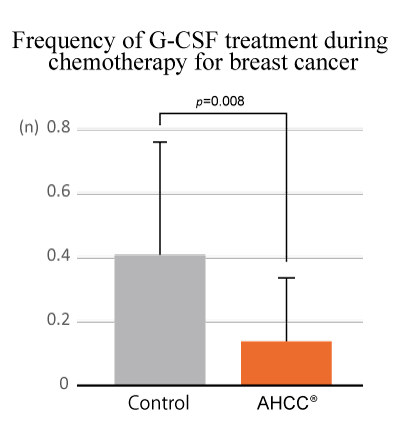
Sho Hangai et al., The Journal of Alternative and Complementary Medicine, 19(11): 905-910 (2013)
Clinical study
Design: A retrospective cohort study
Subject: 41 patients with breast cancer
Groups: AHCC® (n=18) and Control (non-AHCC®) (n=23)
Dose: AHCC® 3 g/day
Endpoints: Adverse event rate, neutrophile count, and average number of granulocyte colony-stimulating factor (G-CSF) usage
Results
Supplementation of AHCC® significantly improved neutropenia and reduced the use frequency of G-CSF, suggesting that AHCC® might contribute to continue adjuvant chemotherapy with an effective treatment intensity by alleviating its side effects.
RESEARCHSuppression of cancer stem cell*2 proliferation
AHCC® suppressed proliferation of cancer stem cells and increased the number of substances that inhibit infiltration*3 and metastasis*4 of cancer
Although cancer is in remission attributable to cancer treatments, a risk of recurrence never disappears. The risk is one of the biggest stresses for patients and their family.
This study investigates if AHCC® inhibits the proliferation and metastasis of cancer stem cells and the progenitor cells, which induce recurrence and metastasis.
The results demonstrate that AHCC® reduces the number of mammospheres caused by tumor cell growth and increases factors to suppress cancer metastasis, expecting that supplementation with AHCC® contributes to decreasing cancer stem cells and preventing cancer recurrence and metastasis.
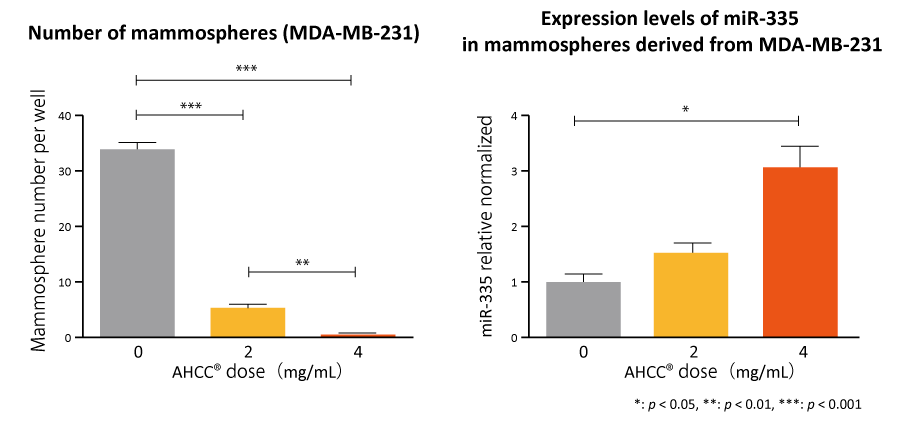
Émilie A Graham et al., Cancer Biology & Therapy, 18(10): 765-774 (2017)
Non-clinical study (in vitro)
Cells: Cancer stem cells and mammospheres derived from cancer stem cells
Concentrations: AHCC® 0, 2, and 4 mg/mL
Evaluation: Formation number of mammospheres and expression level of miRNA
Results
AHCC® significantly reduced the number of mammospheres and upregulated miR-335 expression, suggesting its possibility to contribute in prevention of cancer recurrence and metastasis.
Data are shown as means±standard error of the mean (SEM).
*2:Cancer stem cell(s): cancer cells with abilities of self-replication and tumor formation, and resistance to anti-cancer drugs and radiation.
*3, 4:Infiltration and metastasis: Movements of cancer cells spreading to neighboring tissues and migrating to other organs through blood flow and other factors
RESEARCHCombination with Cancer Immunotherapy
AHCC® is expected to promote the anti-tumor effects of immune checkpoint inhibitors.
Immune checkpoint inhibitors are drugs that block checkpoints on immune cells from binding partner proteins and enable them to attack cancer cells. This study examined if AHCC® enhances the function of immune checkpoint inhibitors. From the results that cancer immunotherapy in combination with AHCC® suppressed the increase in tumor size more effectively than without AHCC®, it is expected that AHCC® supplementation can enhance the anti-tumor effect of immune checkpoint inhibitors and assist in increasing therapeutic efficacy.
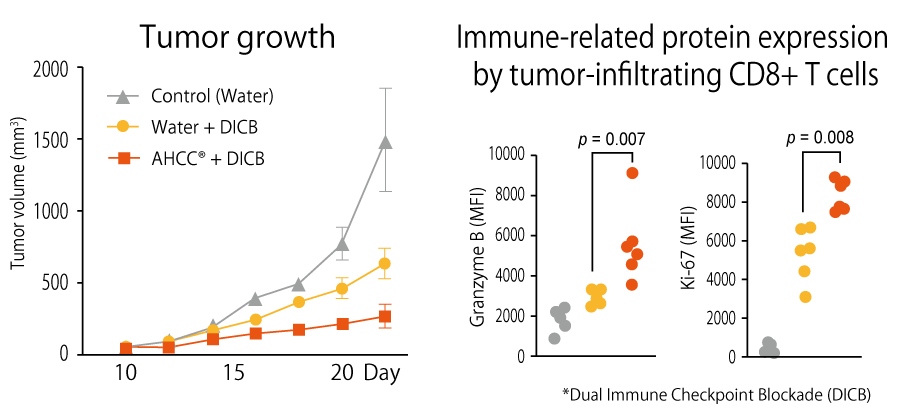
Insoo Kang (Yale University, USA), et al., Frontiers in Immunology, 13: 875872 (2022)
Non-clinical study (in vitro)
Animal: mice inoculated with mouse colon cancer cell MC38 at day 0.
Groups: AHCC® (n=6) and control (n=6)
Dose and period: AHCC® (18 mg/mouse) from day 3 to day 21~27.
anti-mouse CTLA-4 antibody (50 mg/mouse) at day 14 and 17
anti-mouse PD-1 antibody (50 mg/mouse) at day 14 and 17
Evaluation: tumor volume, and expression levels of tumor-infiltrating CD8+ T cells-related molecules
Results
It was revealed that the combination of AHCC® and immune checkpoint inhibitors activated T cells and increased expression of the cytotoxic molecule granzyme B and the cell proliferation marker Ki-67 by tumor-infiltrating CD8+ T cells. As a result, increase in tumor volume could be suppressed effectively.